
Tandem Diabetes Care (Nasdaq:TNDM) today announced the commercial launch of its Mobi insulin patch pump in the United States.
The San Diego-based company says Mobi, which is fully controllable from a mobile app, is the world’s smallest durable automated insulin dosing (AID) system. It received FDA approval in July 2023 for diabetic patients aged 6 years and older.
Tandem announced today that it has begun accepting orders and shipping Mobi to eligible U.S. customers.
The Mobi features a 200-unit insulin cartridge and an on-pump button, providing an alternative to phone control for insulin bolus administration. It is less than half the size of the existing tandem pump system, the t:slim X2 pump. Mobi can fit into your coin pocket, clip onto your clothing, or attach to your body with an adhesive sleeve.
This system features the same Control-IQ technology as the acclaimed t:slim X2. Control-IQ is an advanced hybrid closed-loop automated insulin delivery feature that predicts and prevents hyperglycemia and hypoglycemia. This leads to improved cruising range throughout the day and night.
“This small wearable pump has exceeded user expectations from our early access program, and we are excited to bring this exciting new technology to more people in the diabetes community,” said John Sheridan, President and CEO of Tandem. I think so.” “With this launch, we are executing on our strategy to deliver a differentiated portfolio of durable insulin pumps, offering new options for choice and wearability.”
Other features of the Tandem Mobi system
Mobi is compatible with all existing Tandem brand infusion sets manufactured by Convatec. This includes a new 5-inch tube option made specifically for Mobi. Infusion sets allow the user to temporarily disconnect from the pump for convenience. The company offers more than 30 injection site and tube length combinations.
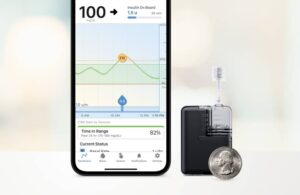
Users can control the Mobi pump through a compatible iPhone with a mobile iOS application. The system is currently compatible with the Dexcom G6 continuous glucose monitor (CGM), and Tandem plans further integration. First, the company expects his G7 integration to take place in the second quarter of this year, followed by Abbott FreeStyle Libre 3.
Tandem has recently made great strides in CGM integration, becoming the first company to combine automated insulin dosing with Dexcom’s latest technology when it integrated G7 with t:slim X2 in December. The company then became the first company to add Abbott’s CGM to his AID technology by integrating his FreeStyle Libre 2 Plus into the t:slim X2.
Additional features of the Mobi include water resistance for up to 2 hours at a depth of 8 feet. This system has a physical button that allows the user to administer insulin boluses without using his iPhone. Finally, the system uses state-of-the-art wireless charging and features remote software update capabilities.
Tandem plans to process orders on a first-come, first-served basis. Initial shipment will be available to users who meet certain eligibility requirements, including use of G6.


